6-9-2025 The HVAC&R Weekly Edition: Issue #18
How about some help understanding Mass Flow vs. Volume of Flow, and how to get the design capacity from a Chiller that was not selected for Propylene Glycol.
If you were around for my earlier Substack Sessions on Psychometrics (1-22-24 thru 4-22-24), you may have picked up on the idea that Mass Flow and Volume of flow should be viewed differently. It stands to reason that the same might hold true for water; what with it being a fluid as well, and in part that’s fairly accurate.
WITH PUMPS AND BLOWERS SOMETIMES A FLUID IS JUST A FLUID, BUT SOMETIMES…..
When we examine the Specific Heat of a substance we are analyzing its capacity to absorb or reject heat. Water is often used as a basis for several standards in the scientific and mechanical world, Specific Heat is one of those standards. It describes the quantity heat in BTUs that must be added or removed from a substance to change the temperature of one pound of that substance, one degree Fahrenheit.
Anti-freeze solutions, specifically, ethylene glycol, propylene glycol, and brines, are sometimes called secondary coolants. They are often used to lower the freezing point of water. A point to note, glycol solutions are sometimes referred to as brine solutions, so be forewarned you may run into a true NaCl brine solution out there as well.
There is a “penalty” or trade off when arbitrarily adding anti-freeze to water to lower its freezing point. Water, pure water to be accurate, has a Specific Heat value of 1.0. That means adding or removing one BTU to (or from) one pound of water, will cause the temperature to change by 1°F. A Specific Heat value less than 1.0 means its capacity to carry BTUs is reduced by that ratio.
Adding an anti-freeze like propylene glycol to a chilled water system, can have negative effects on its capacity by changing its Specific Heat, its Thermal Conductivity, and its Specific Gravity values.
A SNAP SHOT VIEW HIGHLIGHTING THOSE DIFFERENCES IN THE HX.
Inside the Chilled Water heat exchanger, the R-134a refrigerant and the copper tube values for Specific Heat and Thermal Conductivity remain constant for the designed temperature.
By adding Propylene Glycol, the Specific Heat of the solution is reduced to .895 BTU/Lbs./°F, its Thermal Conductivity which determines how effectively heat is transferred through the solution is reduced to 0.213 BTU/hr.-ft.-°F. (The smaller this number the more resistant to heat transfer a substance is.)
Since we can’t change the refrigerants’ thermodynamic properties, or the size, length, or area of the copper tube, or their exposure to the Glycol solution, we will need to make changes to the solutions rate of flow, and pressure drop through the heat exchanger if its design capacity is to be regained. It’s those changes and the increase in Specific Gravity that makes this necessary.
Let’s go about finding that new flow rate and pressure drop through the heat exchanger to give us the original 950 Tons of cooling.
To calculate the correct flow rate for 45% Propylene Glycol we need to develop our own constants. The first should look familiar from last week our 463 custom number.
The second calculation will give us the customized “24” factor to allow us to find the required G.P.M. using the 463 and this formula we get 25.92 for 45% Propylene Glycol.
With this data we can find the correct G.P.M. for the new Specific Heat, Thermal Conductivity and Specific Gravity. 2,462 G.P.M.
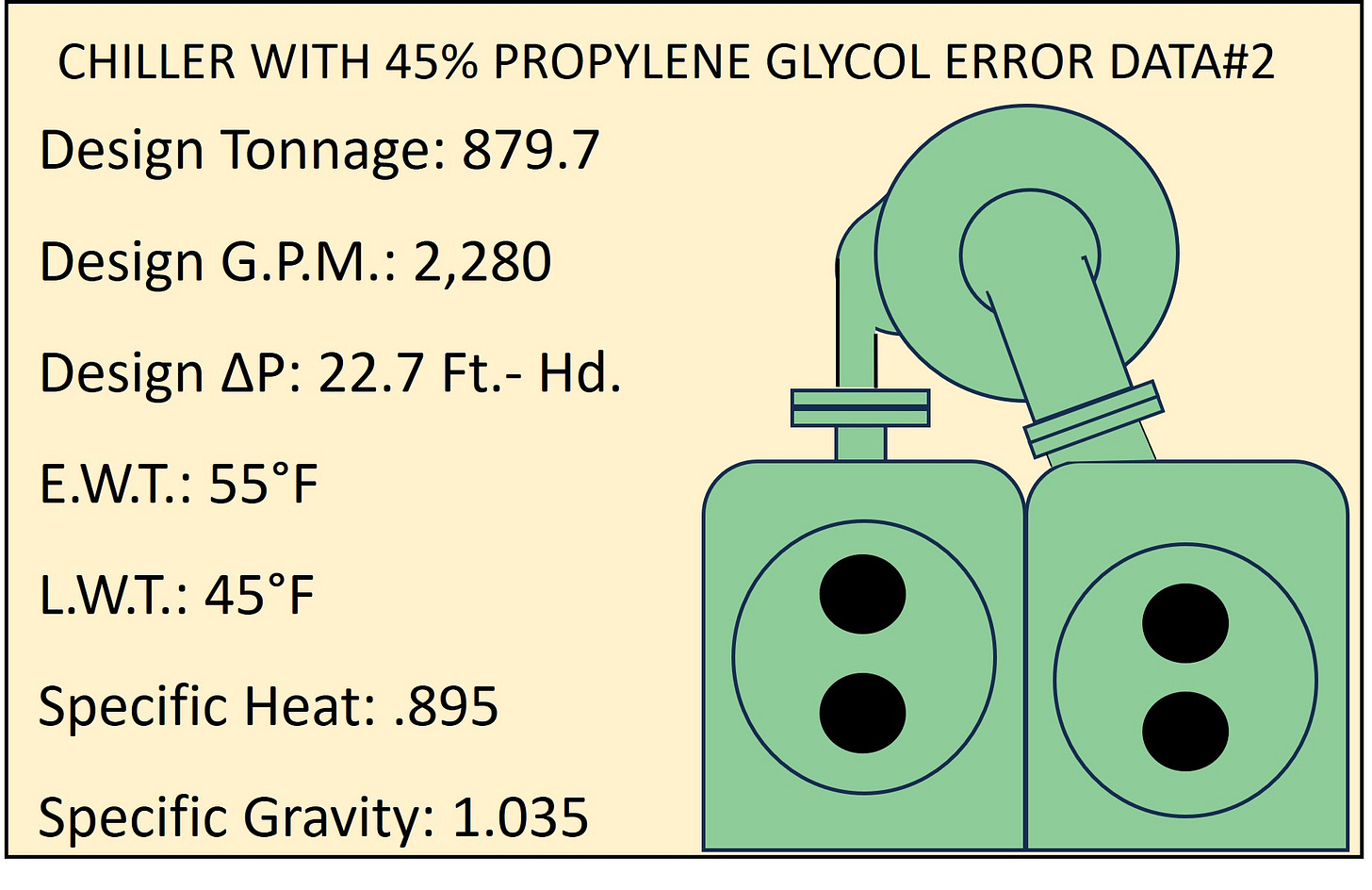
Last thing on our “to do” list for todays Session is to find the corrected ∆Ft.-Hd. To do that we need to use an accurate reference data point for flow and ∆Ft.-hd. when the solution is 45% Propylene Glycol. That data reference point will be the 2,280 G.P.M. and the 22.7 Ft.-hd. we corrected for in last weeks Session.
CORRECTED DATA POINTS FOR 45% PROPYLENE GLYCOL 2,280 G.P.M. AND 22.7 Ft.-Hd. AT 879.7 TONS
Taking those two values and using one of the Pump Affinity Laws (G.P.M. vs. ∆P) we can find the correct Ft.-hd. for the new required G.P.M. of 2,462 G.P.M.
Lets start with the proper Pump Affinity Law or formula:
Plugging in the original data and the newly confirmed G.P.M. of 2,462 we can determine what the proper ∆Ft.-Hd. is to be. Remember that ∆Ft-Hd. changes to the square of the G.P.M.
By setting or adjusting the pressure drop readings to read 26.47 Ft.-Hd. across the evaporator, we can be assured that we will be pumping 2,462 G.P.M. through the barrel and getting the designed 950 Tons of cooling.
The other issue often overlooked is the G.P.M. and pressure drop reading at each chilled water coil and chilled water pump should be adjusted for the change in Specific Heat and Specific Gravity as well. Now can you begin to see the significance of what adding a “little” Glycol to a system can be?
For next week’s Session we will look at the effects the decision to add Propylene Glycol to our Chilled Water system can have on the performance of the centrifugal pump(s). We might even get into working with some pump curves. I can hardly wait!
Until then have a safe week. If you have any questions or comments please feel free to reach out.







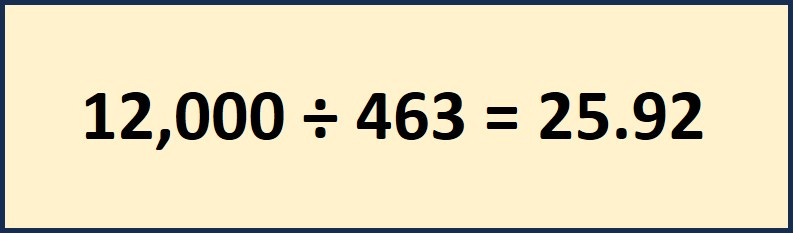
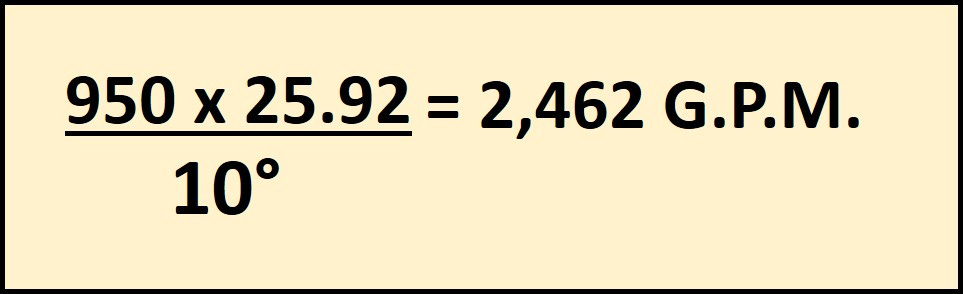




Thanks for the ❤️ Matt.
Thanks for the ❤️ Maren.